
Stop Cancer In Its Tracks
June 24, 2017The Origin of Disease
Science has been in a race to find the holy grail of the origin of disease and the key to anti-aging that would trump all others discovered in the past. It’s becoming more believable to think that all diseases may stem from just ‘the one thing’. After all the body is nothing more than a pyramid of pathways branching off to other important bodily functions that can be affected by different toxins. Any disruption at a certain level of the pyramid can cause a myriad of problems that at first glance may seem to be unrelated to each other, however, share a biochemical connection. The higher up the pyramid that is affected by a particular toxin, the more problems that are manifested.
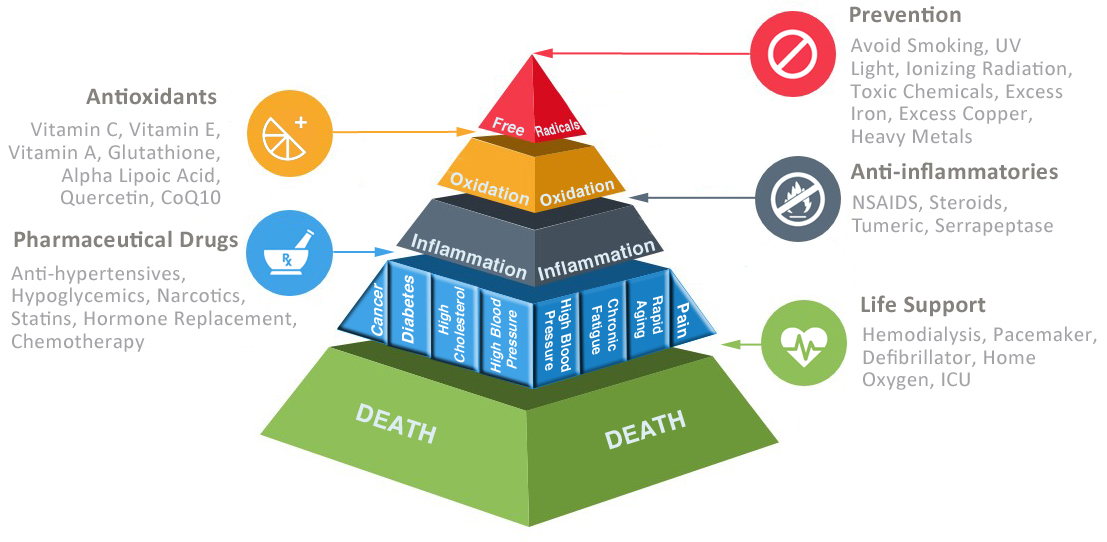
The race to reveal the one thing at the top of the pyramid could mean the end of all diseases and the secret to longevity. Are we there yet? Well, this is debatable depending on who you talk to, however, we are creeping higher and higher up the pyramid and preventing many debilitating and chronic diseases.
As I’ve discussed before, inflammation is pretty close to the top of the pyramid and is the cause of or involved in virtually all diseases. But even inflammation has a cause and can be linked to free radical production or more specifically an imbalance between free radical production and antioxidants. This is a state of oxidative stress.
Oxidative Stress
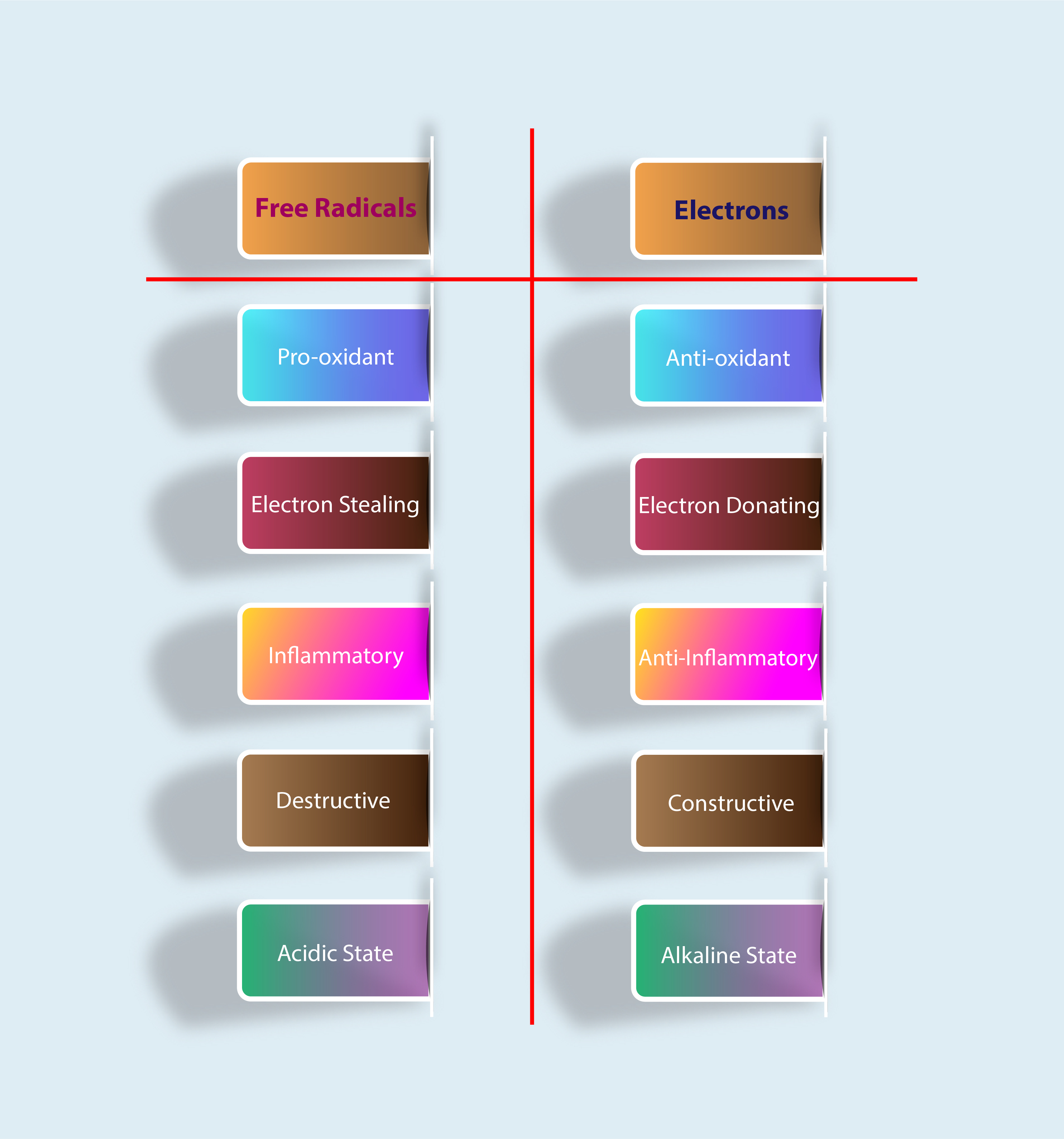
Free radicals are electron-stealing, pro-oxidant, inflammatory, destructive and involved in an acidic state. Electrons, on the other hand, are electron-donating, antioxidant, anti-inflammatory, constructive, and are involved in an alkaline state. So you can say that free radical and electrons are opposites of each other and cancel out when combined. That’s why keeping these elements in a state of balance is important for optimal health.
Free radicals can be damaging because they steal electrons and cause a chain reaction leading to inflammation. Ironically, we need free radicals to live for several reasons. For one, free radicals fuel our immune system in killing pathogens. Additionally, free radicals signal cell proliferation and survival at low concentrations. They also structure the water in the body which is essential for proteins to fold correctly and function optimally. Specific antioxidant enzymes like catalase and superoxide dismutase to reduce too much free radical production intracellularly.
Maintaining Redox Balance
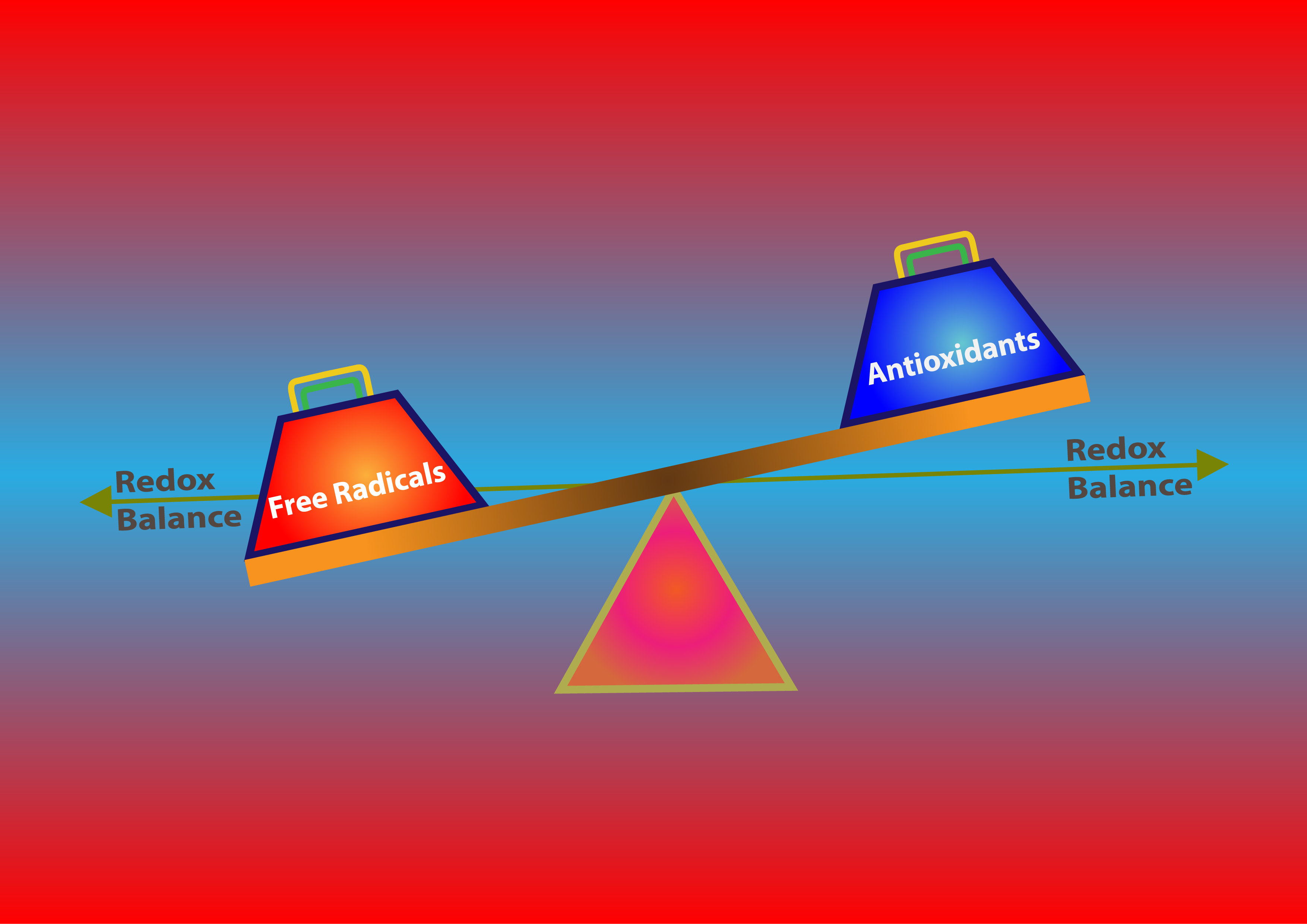
There is a certain balance that needs to be maintained in the body between free radicals and antioxidants. You do not want too much of either. It’s just so easy and effortless to have an over production of free radicals from normal biological processes, toxins, ionizing radiation, and smoking that taking an abundance of antioxidants have been pushed so much. You do need a level of free radical production like for example hydrogen peroxide which is used by the immune system to destroy pathogens. Without it, you wouldn’t survive the week. So, overdoing it with antioxidants may hinder this process. Balance is key.
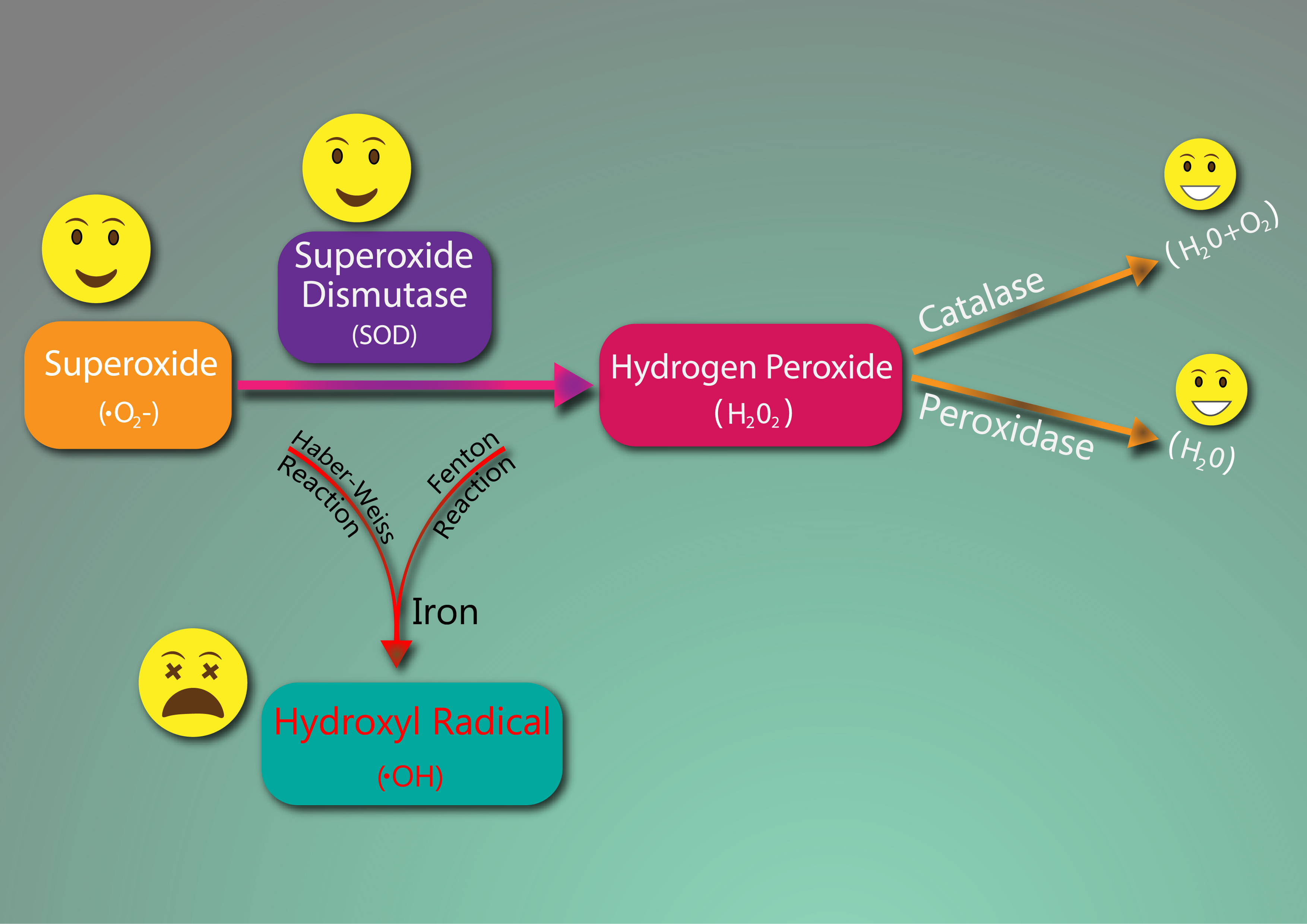
Superoxide and hydrogen peroxide are free radicals made from normal metabolic processes and are relatively benign and unreactive to cellular contents depending on certain conditions. Superoxide is needed in phagocytes which are cells that consume pathogens. The body is protected because superoxide is broken down to hydrogen peroxide by an enzyme called superoxide dismutase (SOD).
Hydrogen peroxide is produced in the mitochondria from normal oxygen metabolism. It’s normal and necessary to have low levels of hydrogen peroxide. For one, it’s needed by immune cells to kill pathogens and it acts as an intracellular signaling molecule which activates cell division, cell differentiation, and cell death. This is important when it comes to cancer. Hydrogen peroxide is broken down by the enzymes catalase and glutathione peroxidase protecting the body from reactions with biological components.
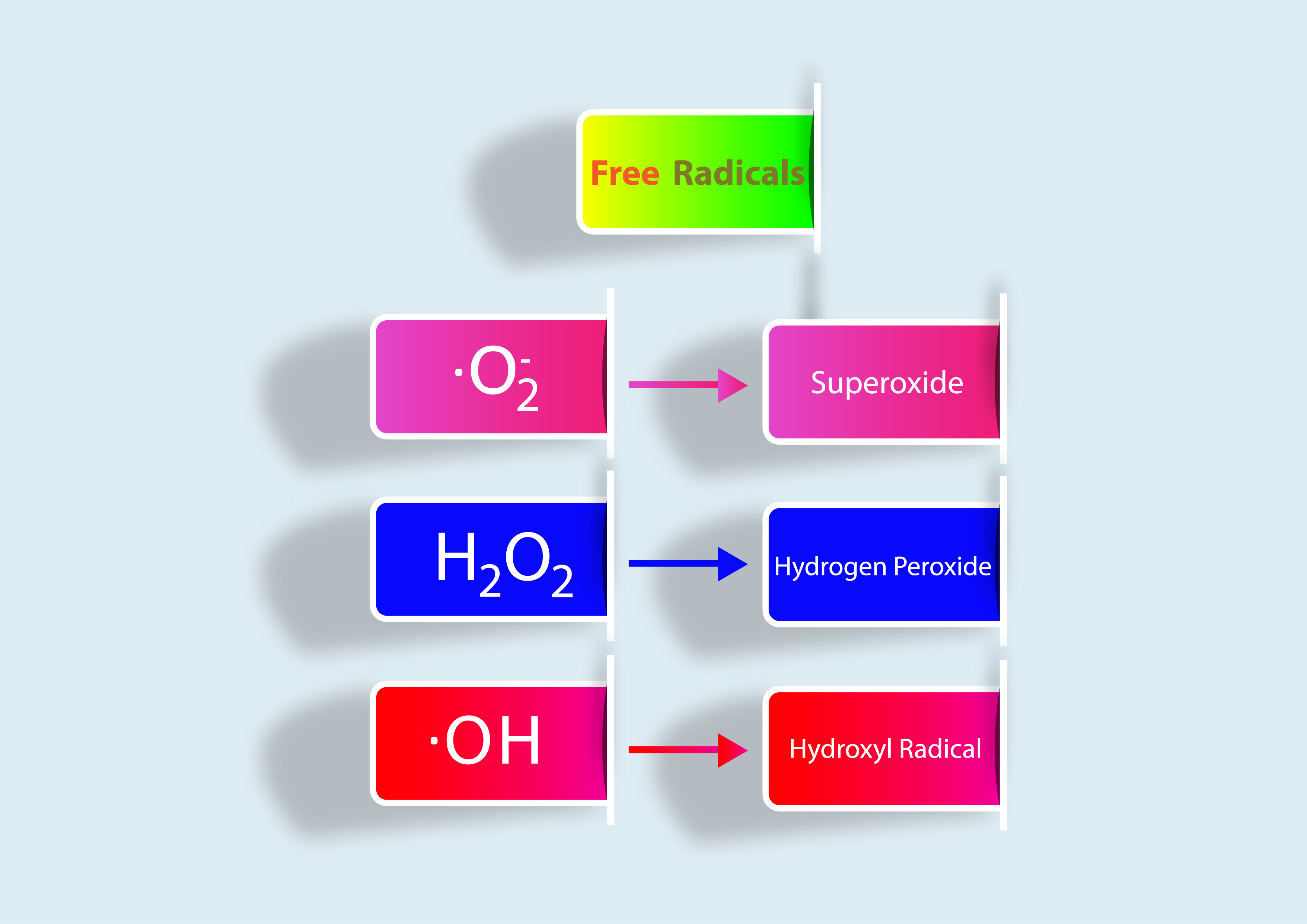
The Role of Iron
Now, this is when the problem starts. Hydrogen peroxide is pretty non-toxic to the body with the right enzymes, HOWEVER (and this is a big however), in the presence of too much iron it can reduce hydrogen peroxide into the lethal, very damaging, and dreaded hydroxyl radical. This radical is highly reactive and oxidizes aka damages ANYTHING it touches. Why is it so damaging compared to the other radicals we’ve just talk about? Well, while superoxide and hydrogen peroxide have special enzymes that break them down in milliseconds, the hydroxyl radical has NO enzyme to break it down and it wreaks havoc on the body uninhibited. This starts a chain reaction of oxidative damage leading to inflammation and chronic disease.
There are only two things that can happen to hydrogen peroxide once it’s produced. It can either be broken down into water by catalase or glutathione hydrogenase or it can react with iron to produce the dangerous hydroxyl radical. The latter is called the Fenton reaction. You might be thinking right now, how does this make any sense? Everyone has iron. You need it. How would you ever avoid this reaction, if you need iron to live?
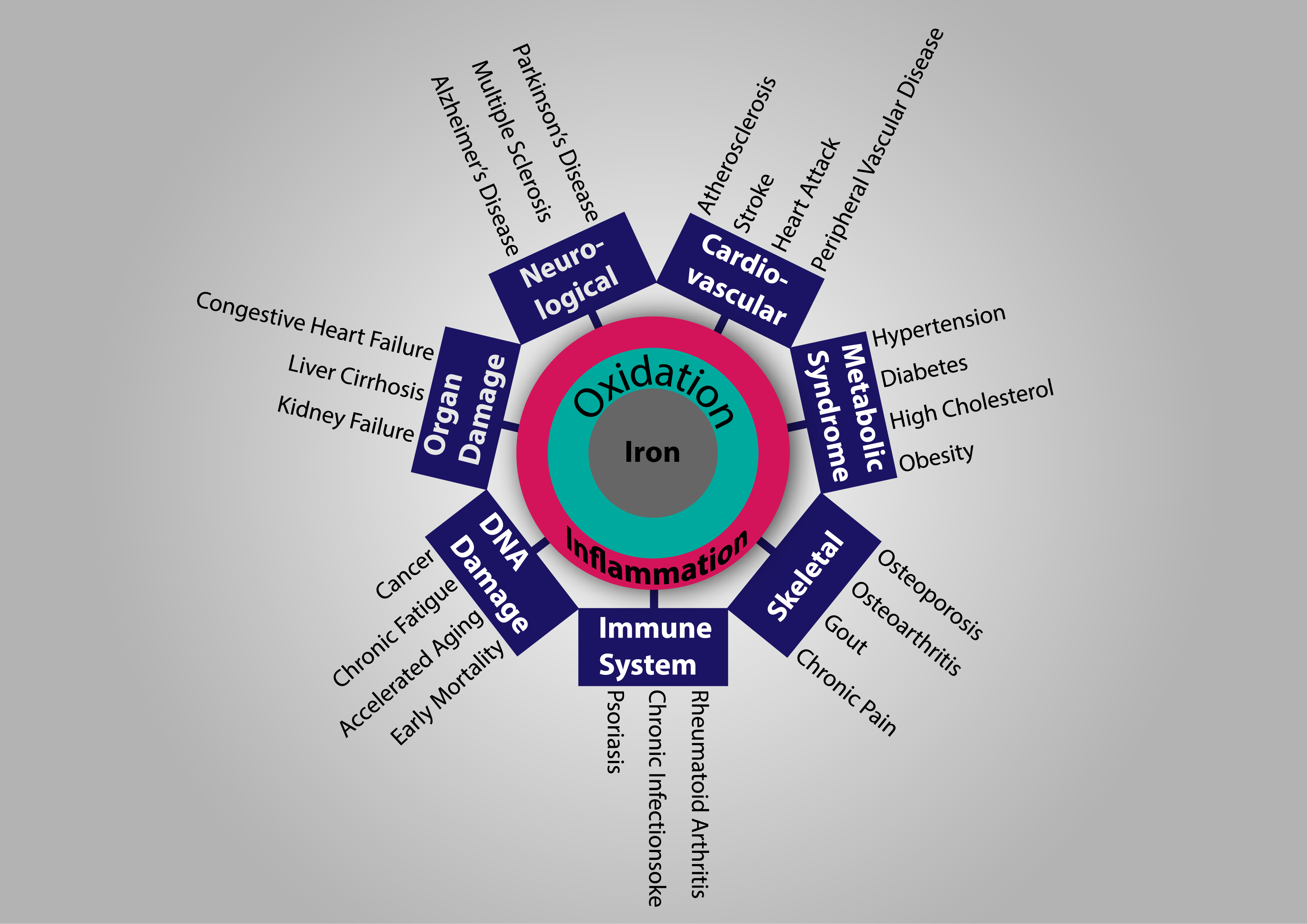
What many do not realize is that while we need iron to live, too much it can actually kill us. Traditional medicine would have us believe that chronic disease, chronic pain, and chronic fatigue is a normal process of aging, however, what’s really happening is that iron is killing us slowly. Iron steals electrons and in the process creates the very destructive hydroxyl (.OH) free radical. This hydroxyl free radical is the worst free radical that can be produced and causes a slew of damage. This is called oxidation aka inflammation aka iron rusting in your body and your body rusting in its own way is called aging.
There are a couple of problems with iron. Some iron we consume may not be bioavailable meaning that our bodies cannot utilize it. If we can’t utilize it, we have to store it somewhere because we cannot easily get rid of it. Also, iron may be what is called poorly liganded which means that it’s loosely bound to a protein or not being bound to any protein at all means you have free iron roaming around which is not a good thing. Metals typically need to be bound to carrier proteins (like ferritin and transferrin) which shield the body from any damage they inflict. This holds true for toxic metals like mercury, lead, and aluminum and why it’s important to chelate these chemicals out. Wherever in the body free iron lands, inflammation and damage will follow. Wherever free iron is, it’s safe to say hydrogen peroxide will also be there since all cells produce it from normal aerobic respiration. Now you have hydrogen peroxide reacting with unbound iron that is being recycled, reduced, and used again resulting in the production of the damaging hydroxyl radical. What’s even worse is that iron will always accumulate if there is no process for its elimination. This is more true for men than women which I will talk more about shortly. Having too much iron may be worse than smoking cigarettes continuously 24/7.
Consequences of Iron Overload
| High Iron levels can cause lipid oxidation which causes an inflammatory state, raising cholesterol levels, leading to atherosclerosis and cardiovascular disease. |
| High iron levels in the brain lead to free radical production in the brain which eventually leads to neurodegenerative diseases like Alzheimer’s and Parkinson’s disease. |
| High iron levels in the cells cause the uncoupling of oxygen metabolism from ATP synthesis leading to decrease energy and chronic fatigue. The decrease in metabolic energy can also lead to cancer. |
| High iron levels make the blood more acidic, causing calcium to spill into the blood from the bones leading to osteoporosis and osteoarthritis. |
| High iron levels lead to bacterial overgrowth and chronic infections. |
| High iron levels cause generalized oxidative damage which leads to generalized inflammation, cellular damage and ultimately accelerated aging. |
| High iron levels are an indicator for early all-cause mortality. |
| High iron levels can overwhelm the pancreas tissue and lead to diabetes. |
| High iron levels in the liver can lead to liver cirrhosis and failure. |
To find out if you’re overloaded, you need to check your serum ferritin level. Ferritin is the intracellular carrier protein for iron. Ideally, you want your ferritin level between 20 – 50 ng/ml. In general, you want to keep your ferritin levels under 75 ng/ml. When you start having levels in the 200s, 300s, 400s you’re going to start noticing a few things:
• Random joint pains and muscles aches.
• Increase in blood pressure and insulin resistance leading to diabetes.
• Hemoglobin A1C level rises or won’t go down.
• Cholesterol, CRP, and ESR which are all markers of inflammation start to rise.
• More frequent colds and localized infections.
• Loss of more magnesium than taken in causing muscle spasms and migraines.
• Hair graying in the 30s, low energy, and brain fog.
• Unexplained cancer.
I took care of a 64 yo female whose ferritin levels were 1,830! This patient had advanced high blood pressure, advanced diabetes, high cholesterol, and end-stage kidney failure receiving dialysis 3 times a week. The following week I had another patient who was a 21-year-old male with a ferritin level of 8,063 and it turned out he had lymphoma. Some may argue that the lymphoma caused the high ferritin, but it’s documented that iron overload can cause cancer. That’s what a ferritin level that high gets you.
You would think that getting a serum iron level would correlate better for iron deficiency or iron overload. Serum iron only measures iron bound to the transferrin protein, and not unbound free iron. Transferrin is a carrier protein that mops up any free iron floating around and transports it through the blood. There is a limit of how much iron it can carry. Normal saturation for transferrin is 25-35%. A higher saturation may suggest iron overload while a lower saturation may suggest iron deficiency. However, you can have iron overload with normal iron saturations levels. Most of the leftover free iron in the blood is taken up by cells through special transporters. A lot of iron may be found in the tissues intracellularly affecting the mitochondria machinery or in the extracellular matrix, and not in the blood.
Bloodletting Makes a Comeback
Men are more affected than women when it comes to iron accumulation. One way to release iron from the body is to remove blood from the body. Either you can donate blood twice a year or do serial phlebotomies. Women naturally expel blood every month through the menstrual cycle. This is one of the reasons why women outlive men because they’ve had 30 to 40 years to release iron during menses. Who would’ve known that bloodletting, an archaic form of treatment for disease, would actually have some utility in preventing chronic disease? Women eventually have the same problems as men after they hit menopause. However, after menopause, their mortality rates catch up with their male counterparts.
Where ‘s the All Iron Coming From?
You may be wondering, where is all this iron coming from? If anything, we keep on hearing people being anemic and having low iron. While this may be true for a select few, the entire population is being treated for low iron. So what we’re seeing here is a case of overcorrection on a mass scale. In an effort to treat worldwide iron deficiency, especially among women, flour and bread products were fortified with iron for more than half century. However, there is an epidemic of iron overload that’s not readily apparent. While iron studies are routine for health screening, ferritin is either not a part of the study or totally overlooked.
The Ferritin Misconception
I was taught in medical school that ferritin was a marker for inflammation and may or may not reflect iron levels depending on when it was drawn. So according to what I learned, if there is inflammation already in the body, it may falsely represent a high iron level. I have a different way of looking at this. The elevated ferritin represents the bioUnavailable iron that’s CAUSING the inflammation and also increases the chances of infection because pathogens need iron to multiply. Traditional medicine interprets it the other way around. It’s like the goose and the egg – which came first? Additionally, you’ll come to find out that ferritin levels rarely decrease but increase over time if there is no way to release iron.
Also, ferritin is an INTRACELLULAR carrier protein for iron. You’re not supposed to see it in the blood in high amounts unless there’s damage going on. It’s similar to monitoring troponin levels during heart attacks or creatinine kinase levels during skeletal muscle damage. Liver enzyme levels are monitored to detect liver damage when cholesterol-lowering drugs are being used or pancreatic enzymes to detect pancreatitis. These proteins are being released because of cell rupture and damage. If your ferritin level is elevated, something’s not right. Don’t expect your physician tell you otherwise. Current reference ranges show that having ferritin levels around 200-300 is normal, but evidence suggests that damage is happening right under your nose and you don’t even know it.
The key thing that I want you to understand is that most ferritin being released into the circulation doesn’t have any iron bound to it! Many research articles support this point. So you have an iron carrier protein, which is supposed to protect the body from iron oxidation, that does not have any or very little iron in it! So the question remains, where did all that iron go? All that free iron is floating around the body unbound and unusable to the body and is wreaking havoc on the body. What’s worse is that most of that iron is absorbed by cells where it’s damaging intracellular organelles.
Detoxing Iron
Now let’s talk about how we can get rid of excess iron. The first thing you want to do is bloodlet or donate blood. You should donate blood, especially men, twice a year. Also, serial phlebotomies can also be used to remove blood regularly. Next, you will want to take an iron chelator that will bind iron and remove it from the body. While there are some harsh iron chelators like deferoxamine that need to be used under a doctor’s supervision, I recommend using a natural iron chelator like inositol-6-phosphate (IP6). Curcumin may also help chelate iron. Quercetin and CoQ10 are also beneficial in getting rid of iron. Apple cider vinegar every day will help prevent iron from oxidizing in your body. Dropping your ferritin level 100 points will have dramatic effects in reducing your inflammatory markers like C-reactive protein and erythrocyte sedimentation rate. You may even see a dramatic drop in your cholesterol levels. Wouldn’t it be nice not having to take a cholesterol-lowering drug for the rest of your life?
My recommendation is to add the ferritin to your routine blood work as screening and prognostic tool. I believe that high iron is one of the main reasons for some of the most prominent chronic conditions we see today like hypertension, high cholesterol, diabetes, arthritis, cancer, chronic pain, and chronic fatigue. Iron causes the inflammation, and inflammation starts the pathway to all these other chronic conditions. If you can stop the domino effect upstream and early in the process you can save yourself a lot of pain and heartache. There’s a 5% chance that your physician will know anything about this and may dismiss it. It will be your job to educate them and take control of your own health.
Resources
- Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases; BMC Medical Genomics – 2009 Jan :1-79
- Total and Cause-Specific Mortality by Moderately and Markedly Increased Ferritin Concentrations: General Population Study and Metaanalysis; Clinical Chemistry – 2014; Vol. 60(11):1419–1428
- A diagnostic approach to hyperferritinemia with a non-elevated transferrin saturation; Journal of Hematology – 2011; Vol. 55:453-458
- Non-transferrin-bound iron: a promising biomarker in iron overload disorders; Ned Tijdschr Geneeskd – 2013; Vol. 157(49):A6258
- Body iron metabolism and pathophysiology of iron overload; Int J Hematol – 2008; Vol. 88:7–15
- The Fascinating but Deceptive Ferritin: To Measure It or Not to Measure It in Chronic Kidney Disease?; Clin J Am Soc Nephron – 2006; Vol. 1: S9–S18
- Blood donation, body iron status and carotid intimal-media thickness; Atherosclerosis – 2008 Feb; Vol. 196(2):856-62
- Macrophage foam cell formation during early atherogenesis is determined by the balance between pro-oxidants and anti-oxidants in arterial cells and blood lipoproteins; Antioxid Redox Signal – Winter 1999; Vol. 1(4):585-94
- Macrophage-mediated cholesterol handling in atherosclerosis; J. Cell. Mol. Med. – 2016; Vol. 20( 1): 17-28
- Iron loading increases cholesterol accumulation and macrophage scavenger receptor I expression in THP-1 mononuclear phagocytes; Metabolism. – 2005 Apr; Vol. 54(4):453-9
- Iron Together with Lipid Downregulates Protein Levels of Ceruloplasmin in Macrophages Associated with Rapid Foam Cell Formation; J Atheroscler Thromb – 2016; Vol. 23:1201-1211

